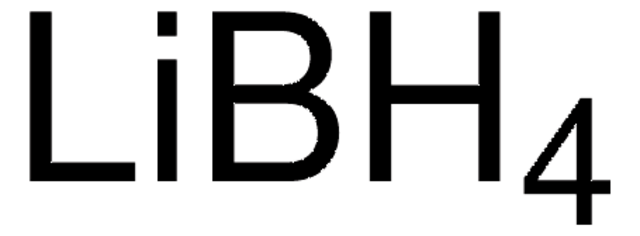222356
Lithiumborhydrid
≥90%
Synonym(e):
Lithiumboranat
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥90%
Form
solid
Eignung der Reaktion
reagent type: reductant
Grünere Alternativprodukt-Eigenschaften
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
mp (Schmelzpunkt)
275 °C (dec.)
Dichte
0.666 g/mL at 25 °C (lit.)
Grünere Alternativprodukt-Kategorie
SMILES String
[Li+].[BH4-]
InChI
1S/BH4.Li/h1H4;/q-1;+1
InChIKey
UUKMSDRCXNLYOO-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Corr. 1B - Water-react 1
Lagerklassenschlüssel
4.3 - Hazardous materials, which set free flammable gases upon contact with water
WGK
WGK 2
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
An article about metal borohydrides as hydrogen storage materials
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.