Comparison of Process-related Impurity Clearance for Membrane Adsorbers and Resin-based Chromatography Technologies
- Study Overview
- Competitive Evaluation
- Salt Tolerance Study
- Phosphate Tolerance Study
- DNA Binding Capacity Study
- Collaborator Data
- Conclusion
Introduction
Strong anion-exchange (Q) chromatography is standard for monoclonal antibody (mAb) purification. This technique removes DNA, viruses, endotoxins and acidic host cell proteins (HCP) from process feed streams in flow-through mode.
As scientists pursue downstream single-use technologies and flexible biomanufacturing due to advancements in cell culture technology and the emergence of cost-sensitive biosimilars, conventional purification technologies present limitations.
Traditional, resin-based chromatography columns are often oversized due to throughput limitations and are ill-suited to flexible manufacturing. Conventional membrane adsorbers offer faster throughput but cannot provide sufficient process robustness due to the low binding capacity. These factors impose challenges on the design of purification schemes for manufacturing of biotherapeutics.
NatriFlo® HD-Q membrane adsorbers overcome these limitations by combining high binding capacity and high flow rates to deliver best-in-class performance in a single-use plug and flow format.
We examine the performance of NatriFlo® HD-Q membrane adsorbers in comparison to currently available quaternary amine resins, membranes and salt- tolerant primary amine membranes. Comparative data is provided using BSA and DNA as benchmark biomolecules.
In addition, the effectiveness and scalability of NatriFlo® HD-Q adsorbers in removing process impurities (HCP, DNA and viruses) from industrial feed streams is also demonstrated.
Study Overview
A typical mAb production platform has three chromatographic steps:
- Capture chromatography
- Intermediate purification
- Polish
Protein A is a popular choice for capture due to its ability to deliver >95% pure product in a single step. After the capture step, mAb is further purified using either one or two additional chromatographic units. Strong anion-exchange (AEX) chromatography is the most popular choice as either the second or thirdchromatographic step due to its proven performance in large-scale mAb purification.
When designing a modern mAb process with AEX as a purification step, the following objectives are typically desired:
- Robust separation performance with high throughput and capacity in flow through mode
- Flexibility in process design (such as positioning of AEX step in the platform, salt tolerance and buffer compatibility)
- Risk-free, scalable and easy operation in single-use format
However, current technologies are limited in their ability to meet all process requirements due to inherent technical limitations, as noted in Table 1.
All the devices were run as per the instructions provided by the suppliers at the recommended flow rate (i.e., membrane adsorbers were run at 10 MV/min, whereas 1 mL prepacked columns were run at 1 CV/min).
The feed sample specifications for the various studies were as follows:
- Salt Tolerance Study: 1 g/L BSA in 25 mM Tris + NaCl buffer, pH 8.0
- Phosphate Tolerance Study: 1 g/L BSA in phosphate buffer, pH 8.0
- DNA Binding Study: 0.1 g/L Herring testes DNA in 25 mM Tris buffer, pH 8.0
Salt Tolerance Study
NatriFlo® HD-Q adsorber maintained superior dynamic binding capacity over a wide range of conductivities in comparison to all membrane adsorbers, including salt-tolerant media (Figure 1 and Table 2). Resin 1–Q demonstrated similar binding capacity to NatriFlo® HD-Q adsorber but suffers from throughput limitations inherent to column chromatography.
The typical residence time for any column at process scale is at least 2 minutes, which puts constraint on the loading capacity of the column. Using this residence time, a typical column would need more than 66 hours to load the feed sample having 5 g/L titer to 10 kg/L capacity. By comparison, NatriFlo® HD-Q adsorber would need only 3.3 hours to load the same feed at 10 MV/min flow rate (or even less if impurity clearance performance is maintained at higher throughputs.)
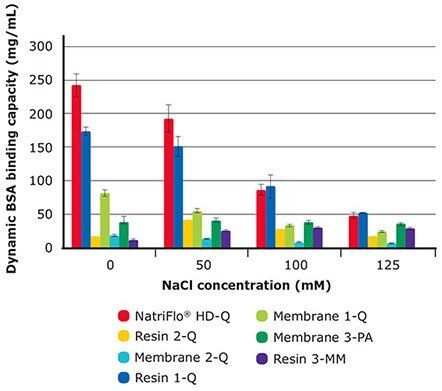
Figure 1.10% BT BSA Binding Capacity: Salt Tolerance
Phosphate Tolerance Study
Phosphate is one of the most popular and widely used buffers for the pH range of 5.8 to 8.0. Natrix® HD-Q membrane can tolerate the presence of phosphate ions unlike other anion-exchange media, whose performance is significantly affected in presence of multivalent buffers.
As illustrated in Figure 2 and Table 4, Natrix® HD-Q membrane achieved more than 50% greater dynamic binding capacity than the closest competitor. This advantage was most pronounced at higher phosphate concentration.
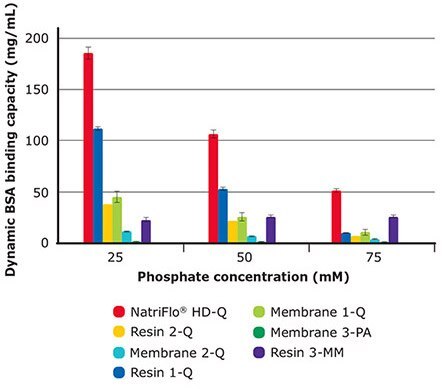
Figure 2.10% BT BSA Binding Capacity: Phosphate Tolerance
DNA Binding Capacity Study
NatriFlo® HD-Q membrane adsorber achieved an average DNA dynamic binding capacity of 27 mg/mL, 59% higher than the next closest competitor (Membrane 1-Q at 17 mg/ mL). None of the other AEX media exceeded 5 mg/mL (Table 5 and Figure 3).
Only the Natrix® HD-Q membrane achieved excellent binding capacity for both protein and DNA without throughput limitation. This is due to the easily accessible, high ligand density of the Natrix® HD membranes.
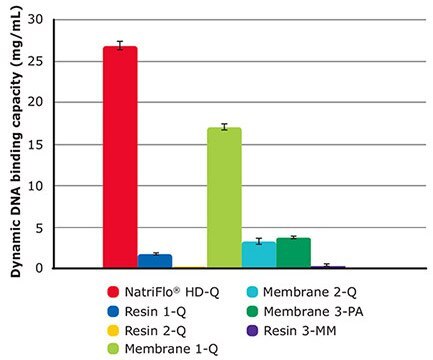
Figure 3.10% BT DNA Binding Capacity
Summary
- The superior dynamic binding capacity of NatriFlo® HD-Q membrane adsorber over a wide range of operating conditions combined with impressive throughput enable simple and robust polishing operations in a single-use format.
- Resin 1-Q achieved good dynamic BSA binding capacity but very low DNA dynamic binding capacity at 1 minute of residence time. In most cases, column chromatography ends up being oversized at process scale due to throughput limitation even though resins offer good protein binding capacity.
- Conventional membrane adsorbers lack the process robustness & design flexibility due to poor binding capacity of media.
Collaborator Data
Several major biopharmaceutical firms (including CMOs) have evaluated the performance of NatriFlo® HD-Q membrane adsorbers in their existing mAb purification platform. The impurity (virus, HCP & DNA) clearance and process scalability data provided in this Application Note represent the achieved performance based on their actual proprietary antibody feed streams.
Collaborator 1: Viral and DNA Clearance
The process targets in this study were to use single-use anion exchange chromatography to reduce DNA levels down to <10 ppb in the mAb feed stream while providing > 4 LRV clearance of two model viruses, xenotropic murine leukemia virus (xMuLV, retrovirus, enveloped, ssRNA, 80-120 nm) and murine minute virus (MMV, parvovirus, non-enveloped, ssDNA, 18-26 nm). The study was conducted at an external testing facility.
The loading capacity of an anion exchange step is often dictated by virus breakthrough. In order to understand the design space for virus clearance, the study was conducted over a wide range of conductivity (5 - 15 mS/cm) and loading capacity (up to 10 kg of mAb/L of membrane). The partially purified mAb feed was diafiltered in 25 mM Tris + NaCl buffer at pH 7.5. The final sample has a 15 g/L titer with 1.3% aggregates, 84 ppm HCP and 83 ppb DNA. The feed sample was appropriately spiked with virus or DNA (CHO genome DNA) just before the experiment to understand virus and DNA clearance.
Summary
The NatriFlo® HD-Q membrane adsorber achieved excellent clearance (≥ 4.8 LRV) of xMuLV virus over a wide conductivity (5 – 15 mS/cm) and flow rate (10 – 25 MV/min) range at 10 kg/L load (Figure 4).
- No xMuLV breakthrough was detected in the flow through for all evaluated scenarios. The processing time at 25 MV/ min was less than an hour.
- Flow independent clearance of xMuLV demonstrates a convection dominant mass transport phenomenon in Natrix® membrane technology.
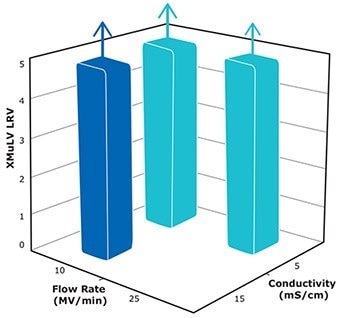
Figure 4.xMuLV clearance at 10 kg/L load
NatriFlo® HD-Q adsorber achieved > 4 LRV MVM clearance with conductivity as high as 10 mS/cm at 10 kg load and 10 MV/min (residence time = 6 seconds) flow rate (Figure 5).
- At 5 mS/cm conductivity, MMV clearance remains constant at 4.8 LRV for up to 10 kg / L load.
- At 3 kg/L load, MMV clearance remains the same (4.8 LRV) from 5 to 10 mS/cm.
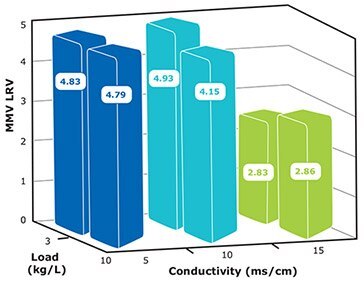
Figure 5.MMV clearance at 10 MV/min flow rate
The data reveal excellent clearance of both viruses at very high mAb loads over a broad range of conductivities with residence time in the order of a few seconds. The high binding capacity of the membrane combined with excellent flow properties and improved salt tolerance provides great process economy without sacrificing the process robustness or design flexibility.
In addition, with the same feed material NatriFlo® HD-Q adsorber demonstrated > 2.9 LRV DNA clearance (from 612 ppb to < 0.7 ppb) at 10 kg/L load (as measured by qPCR assay).
Collaborator 2 - Gallus BioPharmaceuticals: HCP Clearance & Scalability
Gallus BioPharmaceuticals (St. Louis, MO) evaluated lab-scale NatriFlo® HD-Q membrane adsorbers for their ability to remove acidic host cell protein (HCP) at up to 10 kg/L load.
Sample: 8 g/L Protein A purified mAb in 20 mM phosphate + 100 mM NaCl, pH 7
Flow rate: 10 MV/min (residence time – 6 seconds)
The sample feed contained 120 ppm HCP. Figure 6 illustrates that NatriFlo® HD-Q adsorber achieved excellent HCP clearance with no sign of increasing breakthrough, even at 10 kg/L load. Testing was not continued beyond 10 kg/L because of limited supplies of the Protein A purified mAb feed. (Data provided courtesy of Gallus BioPharmaceuticals.)
Gallus also evaluated NatriFlo® HD-Q adsorber for HCP removal across a range of process scales (HD-Q Recon for lab scale; HD-Q Pilot and Process 150 for GMP manufacturing). As seen in Figure 7, excellent and consistent HCP clearance from lab to process scale was achieved, even in phosphate buffer. While the NatriFlo® HD-Q Recon device was tested up to 10 kg/L (Figure 6), the Pilot and Process 150 devices were tested only up to 3 kg/L load due to limited supply of Protein A purified mAb.

Figure 6.HCP Reduction Performance at Lab Scale for 10 kg/L Load
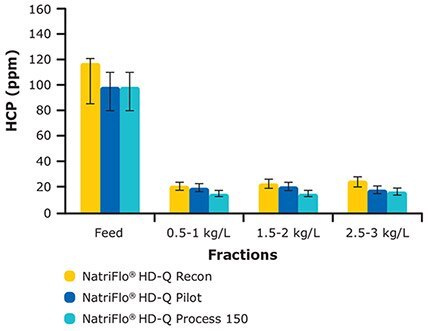
Figure 7.Linear Scalability
Conclusion
NatriFlo® HD-Q adsorber delivers improved economy & greater process design flexibility
As described in the preceding data and discussion, NatriFlo® HD-Q membrane adsorbers represent a new class of chromatography tool that delivers enhanced process flexibility and robustness, with cost and risk reduction benefits associated with single-use technology.
1. Improved salt tolerance and wider buffer compatibility
- Superior dynamic BSA binding capacity in the presence of high salt or phosphate concentrations
2. High loading capacity without compromising process robustness
- Excellent virus (xMuLV & MMV) and DNA clearance from partially purified mAb sample for load as high as 10 kg/L over a broad design space
- Excellent HCP clearance with no sign of increasing breakthrough even at 10 kg/L load
3. Simple scale-up
- Excellent and consistent HCP clearance from lab to process scale
4. Truly single-use plug & flow format
- No column packing, qualification, cleaning, storage, and associated validation
- No risk of cross-contamination
* Process robustness is defined as ability of the process to tolerate variability in operating parameters such as pH, conductivity, buffer type & concentration, impurities in load
** Process design flexibility is defined as ability of the process to tolerate broad range of conditions and flexibility to change the order of unit operations in the purification process
To continue reading please sign in or create an account.
Don't Have An Account?