Biginelli Reaction
The Biginelli Reaction (Figure 1) is an acid catalyzed, threecomponent reaction between an aldehyde, b-ketoester, and urea that produces tetrahydropyrimidones, which have potential pharmaceutical applications.
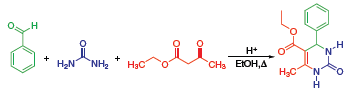
Figure 1.
This reaction was first reported in 1893 and has increased interest because of the final products close structural relationship to the clinically important dihydropyrimidines.1 These compounds are known to show biological activities such as antiviral, antitumor, antibacterial, anti-inflammatory, and more recently, antihypertensive agents.2
The mechanism for this reaction is believed to first be the condensation between the aldehyde and the urea. This produces an iminium intermediate that is the electrophile for the nucleophilic addition of the ketoester enol. The ketone carbonyl that is formed then goes under a condensation reaction with the NH2 of the urea. This gives the cyclized final product.
References
To continue reading please sign in or create an account.
Don't Have An Account?